Covid Vaccine Janssen Fda, Fda Grapples With Timing Of Booster For J J Covid 19 Vaccine Ekathimerini Com
SAHPRA registered the Covid-19 Vaccine Janssen on 31 March 2021 with conditions. Effective April 23 2021 CDC and FDA recommended that use of the JJJanssen COVID-19 Vaccine resume in the United States.
Answers to frequently asked questions about FDAs Emergency Use Authorization of the Janssen COVID-19 Vaccine manufactured by Janssen Biotech Inc a Janssen Pharmaceutical Company of Johnson.

Covid vaccine janssen fda. The Pfizer vaccine is FDA-approved and its booster doses authorized for special groups. Theyll also discuss mixing and matching vaccines. A joint media briefing with the US.
Food and Drug Administration FDA has issued an Emergency Use Authorization EUA to permit the emergency use of the unapproved product Janssen COVID-19 Vaccine for active immunization to prevent COVID-19 in individuals 18 years of. COVID-19 Vaccine Janssen is a vaccine used for preventing COVID-19 caused by. On February 27 the Food and Drug Administration FDA granted emergency use authorization for Johnson Johnsons COVID-19 vaccine for people ages 18 and older.
The Janssen Johnson Johnson COVID-19 vaccine. How Well the Vaccine Works. Advisory committee members say clinical data proves the second shot should be considered part of the primary series while Janssen wants it called a single-shot vaccine with a booster.
Janssen Gets US FDA Panel Nod For Additional COVID Vaccine Shot But Is It A Booster. Importantly the FDA has. The FDAs Vaccines and Related Biological Products Advisory Committee voted to recommend a booster for all recipients of the JJ Janssen.
That FDA advisory panel vote came a day after the same group of independent vaccine experts recommended a third dose of Modernas COVID-19 vaccine for older and higher-risk adults as well as certain workers. Report a Janssen COVID-19 Vaccine Product Quality Complaint Report a Product Quality Complaint to Janssen at 1-800-565-4008 toll free or 1-908. The staff of the Food and Drug Administration on Wednesday struck a more favorable tone on Johnson Johnson Covid-19 booster shots saying there.
The US Food and Drug Administration FDA has authorized emergency use of Janssen COVID-19 Vaccine to help prevent infection with SARS-CoV-2. FDA Authorizes Single-Shot COVID-19 Vaccine. That same population also has been recommended to receive a third dose of the Pfizer shot to ensure better protection against infection.
FDA and CDC Lift Recommended Pause on Johnson Johnson Janssen COVID-19 Vaccine Use Following Thorough Safety Review Agencies Underscore Confidence in Vaccines Safety and. Janssen COVID-19 vaccine is an unapproved product which is under investigation for use as active immunization against COVID-19. The JJ vaccine which is easier to store and deliver will help speed up the coronavirus vaccine rollout.
Media reports show FDA has delayed its decision to authorize the Moderna COVID-19 vaccine for emergency use in adolescents ages 12 to 17. Centers for Disease Control and Prevention and the US. People had the most protection 2 weeks after getting vaccinated.
The Janssen COVID-19 Vaccine has not been approved or licensed by FDA but has been authorized for emergency use by FDA under an EUA for active immunization to prevent Coronavirus Disease 2019 COVID-19 in individuals 18 years of age and older. However women younger than 50 years old especially should be made aware of a rare risk of blood clots with low platelets after vaccination and they should know about other available COVID-19 vaccine options for which this risk has not been seen. EMERGENCY USE AUTHORIZATION EUA OF THE JANSSEN COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019 COVID-19 The US.
Food and Drug Administration FDA. 119 rows The FDA issues a statement regarding the Janssen COVID-19 vaccine issues. The registration was done in terms of Section 15 6a of the Medicines and Related Substance Act 101 of 1965.
The Covid-19 Vaccine Janssen is an adenovirus type 26 vectored vaccine indicated for active. The JJJanssen COVID-19 Vaccine was 663 effective in clinical trials efficacy at preventing laboratory-confirmed COVID-19 infection in people who received the vaccine and had no evidence of being previously infected. Under an Emergency Use Authorization EUA for active immunization to prevent coronavirus disease 2019 COVID-19 caused by severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 in individuals 18 years of age and older.
Johnson Johnson has asked the FDA to authorize booster shots for its coronavirus vaccine but has left it up to the FDA and the US Centers for Disease Control and Prevention to. The Janssen COVID-19 Vaccine has not been approved or licensed by FDA but has been authorized for emergency use by FDA under an EUA for active immunization to prevent Coronavirus Disease 2019 COVID-19 in individuals 18 years of age and older. An FDA expert panel is meeting next week to review Moderna and JJ booster doses.
Today the FDA is announcing revisions to the vaccine recipient and vaccination provider fact sheets for the Johnson Johnson Janssen COVID-19 Vaccine to. The FDA has authorized the emergency use of the Janssen COVID-19 Vaccine to prevent COVID-19 in individuals 18years of age and older under an Emergency Use Authorization EUA. FDA continues to work with its partner in vaccine safety surveillance the CDC to monitor reports of GBS following vaccination with the Janssen COVID-19 Vaccine.
SAHPRA update COVID-19 Vaccine Janssen FDA Developments. The Janssen COVID-19 Vaccine has been granted an Emergency Use Authorization EUA by the US. Food and Drug Administration to discuss six reported cases of a.
Food and Drug Administration FDA. The Pfizer Moderna and Johnson Johnson COVID-19 vaccines are available for use in the US. The Janssen COVID-19 vaccine is authorized for use in the US.
The Janssen COVID-19 Vaccine has been granted an Emergency Use Authorization EUA by the US. Norway Finland and Sweden have suspended use of the Moderna vaccine in young people after increased reports of cardiac side effects including inflammation of the heart muscle and the outer lining of the heart.

Booster Shot Of Johnson Johnson Covid 19 Vaccine Recommended By Fda Panel Colorado Newsline
Fda Grapples With Timing Of Booster For J J Covid 19 Vaccine Ekathimerini Com
About Our Ensemble Studies Johnson Johnson
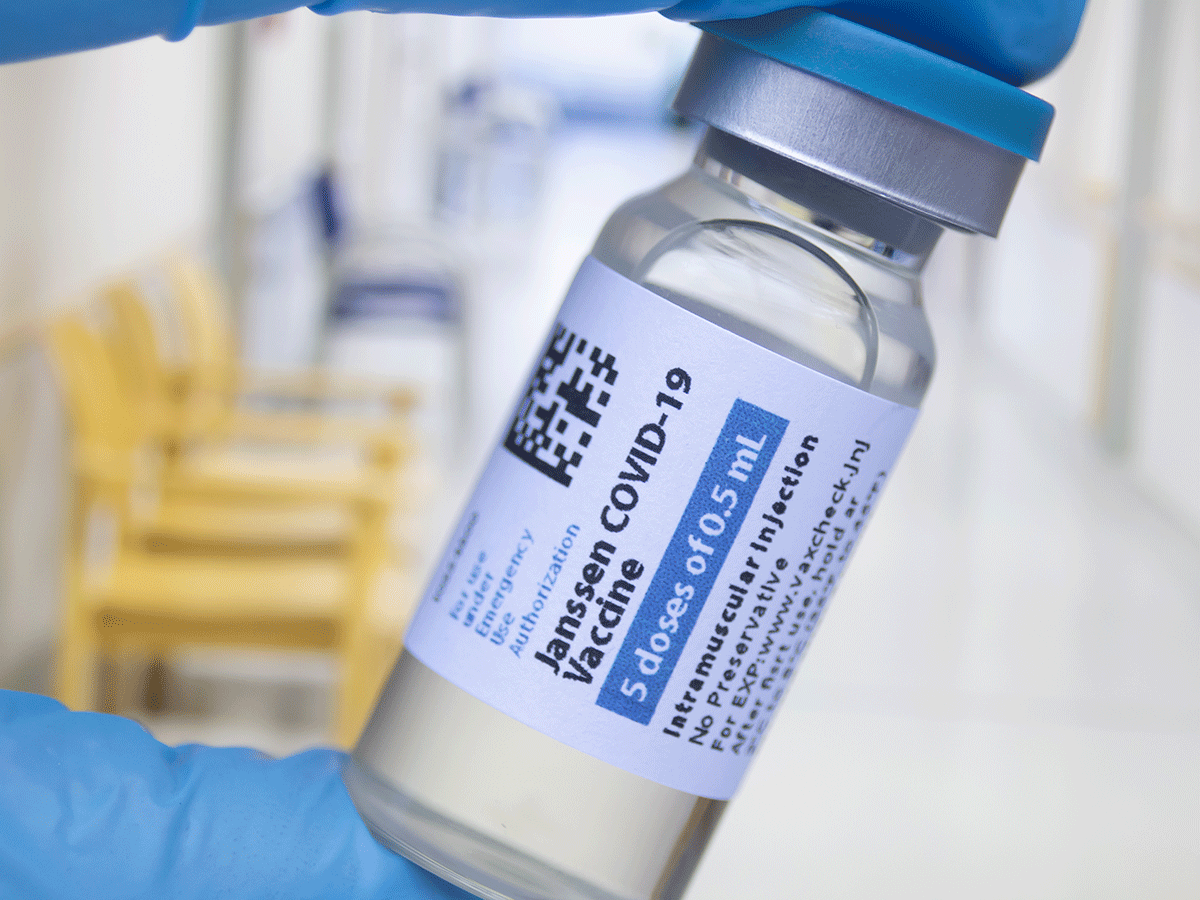
Fda Adcom Unanimously Supports A Booster For Janssen S Covid 19 Vaccine 2021 10 15 Bioworld
Guillain Barre Syndrome Johnson And Johnson Vaccine Fit Increase Risk Of Nerve Disorder Us Fda Warn Bbc News Pidgin

Follow The Fda Advisory Panel Meeting On The J J Covid Vaccine Booster Stat

Fda Grapples With Timing Of J J Covid Vaccine Booster Modern Healthcare
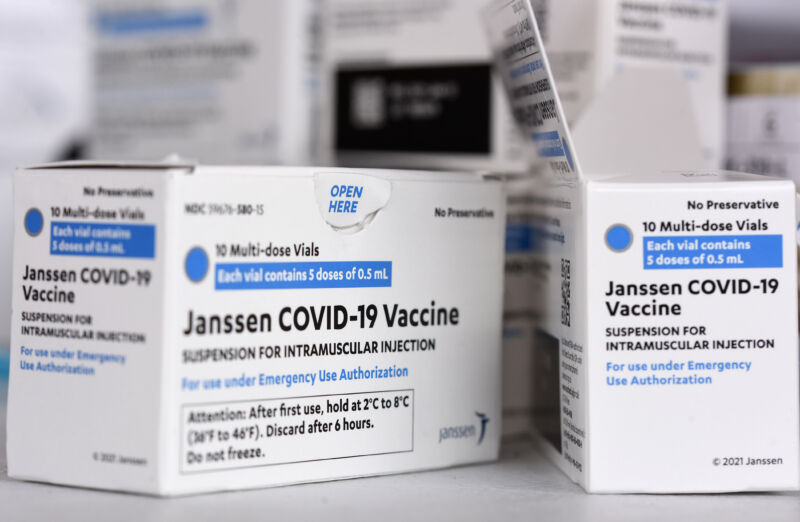
Fda Advisors Recommend J J Boosters For All As Agency Eyes Mix And Match Doses Ars Technica
/cloudfront-us-east-1.images.arcpublishing.com/dmn/2XFTQ5Y75LPPUPSUBYRJZXQF5A.jpg)
Fda Panel Endorses Booster Doses Of Johnson Johnson S Single Shot Covid 19 Vaccine
Fda Approves The Janssen Covid 19 Vaccine For Emergency Use

Fda Grapples With Timing Of Booster For J J Covid 19 Vaccine
J J Covid 19 Vaccine Gets Greenlight From Fda Panel

J J Covid Vaccine Fda To Announce New Warning Related To A Rare Autoimmune Disorder Report Says

Fda Adds J J Covid 19 Vaccine Warning Cidrap

Fda Panel Endorses Booster Shot For J J Covid 19 Vaccine Maryland Daily Record